How Does Ketamine Work?
The Mechanism of Ketamine is Complex and Causes Immediate and Long-term Effects
Ketamine works by blocking N-methyl-D-aspartic acid (NMDA) glutamate receptors, activating α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate receptors, and suppressing and activating gene and protein expression that have both immediate and long-term psychiatric, neurological, cardiovascular, and analgesic effects. Ketamine’s mechanism of action is complex and has a multitude of effects throughout the human body.
Is ketamine’s antidepressant activity due to NMDA blockade?
Studies suggest that no, the antidepressant activity of ketamine is not due to its NMDA blocking properties. Why? Because other NMDA blockers do not demonstrate antidepressant activity. The low trapping NMDA receptor antagonist lanicemine (AZD6765) has shown promise in early small studies, but failed to reach primary antidepressant endpoints in follow-up. The large clinical trials that used 50mg and 100mg doses were extremely costly, leading to the termination of lanicemine’s development.
Ketamine mechanism of action: immediate effects at the molecular level
The immediate effects of ketamine involve the alteration of many cellular mechanisms. Ketamine blocks NMDA channels, blocks neuronal hyperpolarisation-activated cationic currents, blocks nicotinic acetyl-choline ion channels, and enhances the antinociceptive effects of mu-opioid agonists. Ketamine stimulates the L-arginine/nitric oxide/cyclic GMP pathway via neuronal NO synthase to induce peripheral pain-relieving effects. The ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) works by activating AMPA receptors, which may be responsible for ketamine’s anti-depressant effects. Other immediate effects include reduction in cholinergic neuromodulation, inhibition of voltage-dependent L-type calcium channels, alteration of neurosteroid production, and enhancement of the aminergic neuromodulators dopamine and norepinephrine.
Mechanism of action: long-term effects at the molecular level
Ketamine has many long-term effects as well. It suppresses early gene expression at sites of mechanical tissue injury of the following genes zif/268, c-fos, junB, fosB, c-jun, and junD. It also alters the regulation of NMDA receptor phosphorylation and NMDA receptor mRNA expression. Ketamine reduces glial fibrillary acidic protein (GFAP) expression which limits astrocytic and microglial activation. This leads to a reduction in neuropathic pain. It also enhances brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin (mTOR) protein leading to modification to the number and function of synaptic connections.
Ketamine’s antidepressant effects at the anatomical level
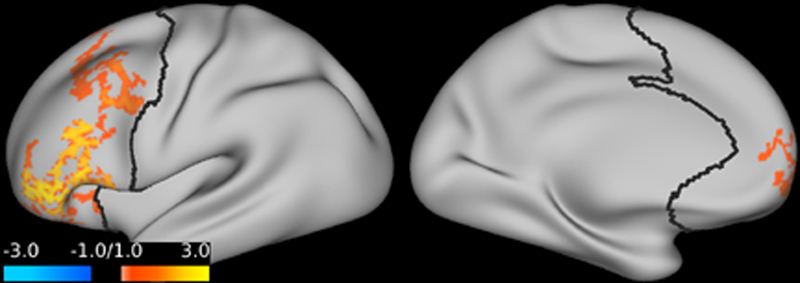
Studies are demonstrating supportive evidence that the prefrontal cortex plays a key role in major depression. Prefrontal cortex global brain connectivity with global signal regression was recently identified as a putative biomarker of major depressive disorder. Emerging evidence repeatedly shows reduced prefrontal cortex global signal regression in major depressive disorder. This abnormality appears to normalize following ketamine treatment. Open-label trials showed significant increases of prefrontal cortex global signal regression 24 hours after ketamine administration in depressed patients. Emerging evidence shows that ketamine increases prefrontal cortex global signal regression during infusion in major depressive disorder, and suggests that ketamine’s rapid-acting antidepressant properties are related to its acute effects on prefrontal connectivity.
Conclusion
Overall, ketamine’s long-term antidepressant effects probably derive from its metabolite action at the AMPA receptor that is somehow tied into its action at the prefrontal cortex. The hypnotic effects are probably caused by a combination of immediate channel blockade of NMDA and HCN1 channels. The more immediate pain-relieving effects of ketamine are probably due to a combination of opioid system sensitisation and aminergic anti-nociception. Whereas its more long-term analgesic effects of inhibition of neuropathic pain relies on a combination of immediate receptor mediated action and initiation of longer lasting cell signalling cascades.
This page reflects ketamine research as of October 2018. We found that some information about ketamine on Wikipedia is outdated.
The top left represents the rapid effects and actions. The bottom right represents more delayed and prolonged effects and actions.
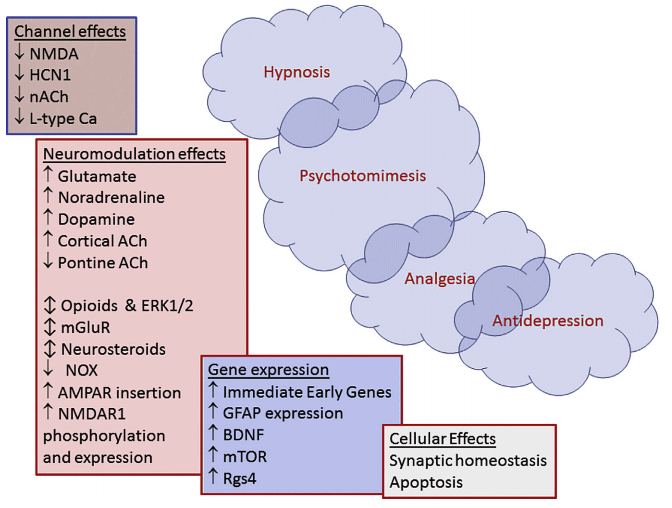
Legend:
NMDA = N-methyl-D aspartate
HCN1 = hyperpolarisation-activated cyclic nucleotide channels
ACh = acetyl choline
nACh = nicotinic acetyl-choline receptors
AMPA = a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
mGluR = metabotropic glutamate receptors
ERK1/2 = extracellular signal-regulated kinases
NOX = NADPH oxidase
BDNF = brain-derived neurotrophic factor
mTOR = mammalian target of rapamycin
Rgs4 = regulator of G protein signalling 4
L-type Ca2 = L-type calcium channels
GFAP = glial fibrillary acidic protein
